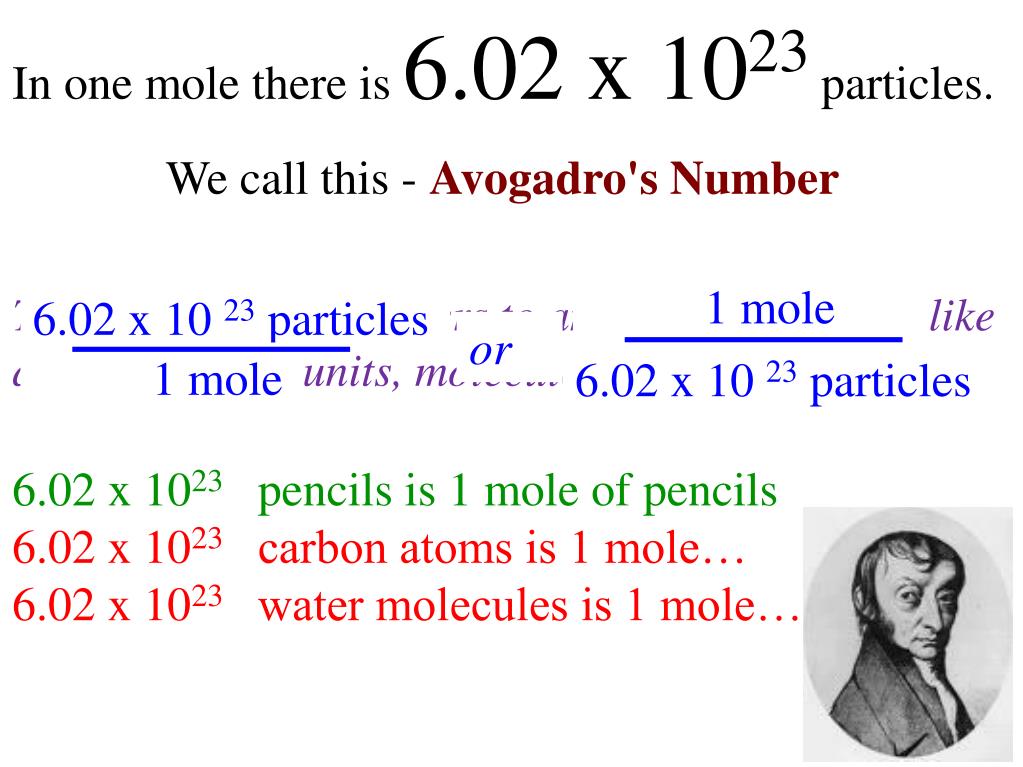
6.02 X 10^23
An Avogadro number of particles 602 x 10 23 particles. ଆମର ମଗଣ ଗଣତ ସମଧନକରକ ବୟବହର କର କରମନସର.
Ppt Chemical Quantities Powerpoint Presentation Free Download Id 4850518
10 to the 23.

. 1g 602 10²³ amu suppose now we want to check number atoms of carbon in 12 grams of carbon because 12 grams is its molar mass 12g 602 10²³ 12 amu on both sides we. A mole of atoms is 602 x 1023 atoms. Chemistry 1 Answer Kai Feb 28 2018 You have one mole of H_2O molecules.
However there is a problem with mole day. For example one mole of iron contains 602 x 10 23 atoms while one mole of water contains 602 x 10 23 molecules. To the nearest orderof magnitude how many moles of atoms are in a large domesticcat.
Theres 23 0s from the. These particles can be either atoms or molecules. The masses of a hydrogen atom.
Mole day is of course 1023. The Avogadro number or constant may be represented by the letter L and when dealing with this number of particles we say that. One mole is 602 x 10 23 Avogadros number.
When 602 x 1023 is multiplied by 91 x 10-31 the product is - 19779193. You know a mole. Exponents first - that means add 23 zeros to 10 then multiply it by 602 What the teacher is explaining is key things to not forget when working with exponents.
One mole represents 60221023 separate entities just like one dozen.

I Got 6 02 X 10 23 Problems But A Mole Ain T One Shir T Shirt Classic

How Many Grams Of Ta Are There In 6 022 X 10 24 Atoms Of Tantalum Socratic

Mole Review Practice Problems

Mole Road Map Overview Examples Of Conversions Expii
![]()
Stream 6 02x10 23 Savage Listen To Podcast Episodes Online For Free On Soundcloud
How Many Grams Of Iron Are In 6 02 X 1023 Atoms Of Iron Quora
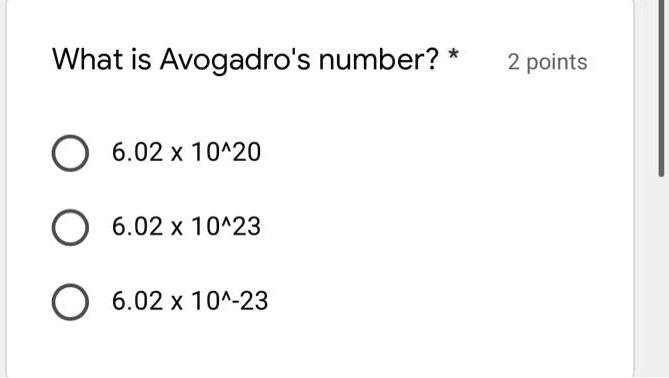
Solved What Is Avogadro S Number 2 Points 6 02 X 10 20 6 02 X 10423 6 02 X 104 23

เลขอาโวกาโดร ต วเลขน ท เด กเคม ต องร จ ก สถาบ นส งเสร มการสอนว ทยาศาสตร และเทคโนโลย สสวท

The Mole Concept Avogadros Constant Mr Carson S Science Page

Avogadro S Constant Surfguppy Chemistry Made Easy For Visual Learners

Chemistry Mole Sample Kit Purchase A Mole Element Project For Your Chemistry Class Experiments At Teachersource Com
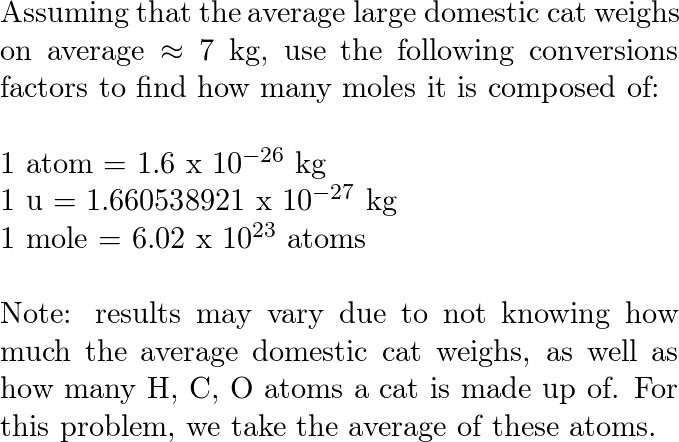
A Mole Of Atoms Is 6 02 10 23 Atoms To The Near Quizlet

How To Convert Moles To Molecules With Examples Science Trends

Problems 4 6 Convert The Measurements Into Moles 4 9 03 X1023 C Atoms 5 2 44x1023 H2o Molecules Brainly Com

I Got 6 02 X 10 23 Problems But A Mole Ain T One Shir
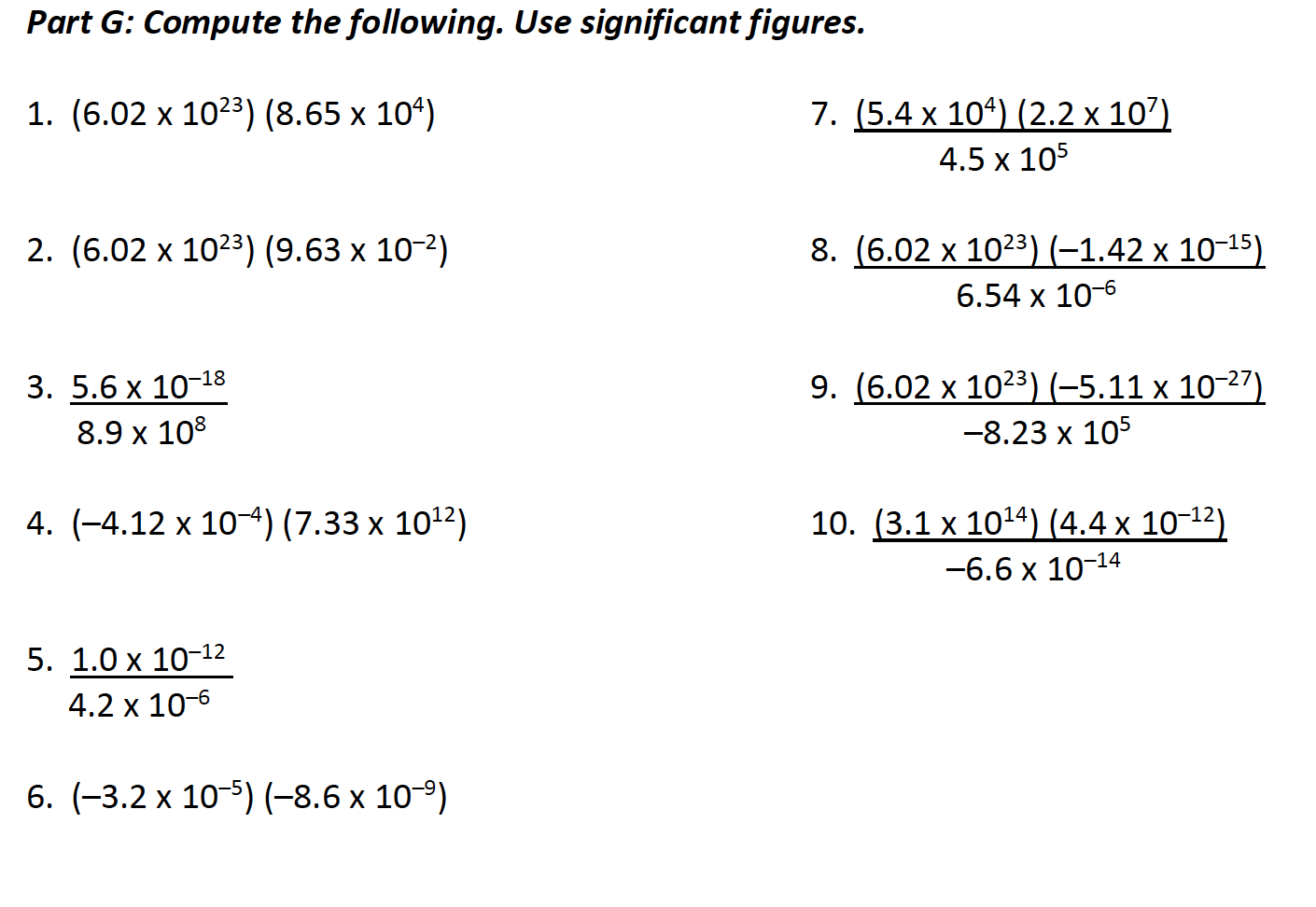
Solved Part G Compute The Following Use Significant Chegg Com

Moles Vs Molecules Is C Correct Because There Are 6 02 X 10 23 Molecules In A Mole So C And D Would Be Automatically Too Small To Even Consider To Count R Chemhelp